Explained: The nationwide rollout of the Pfizer-BioNTech COVID-19 vaccine
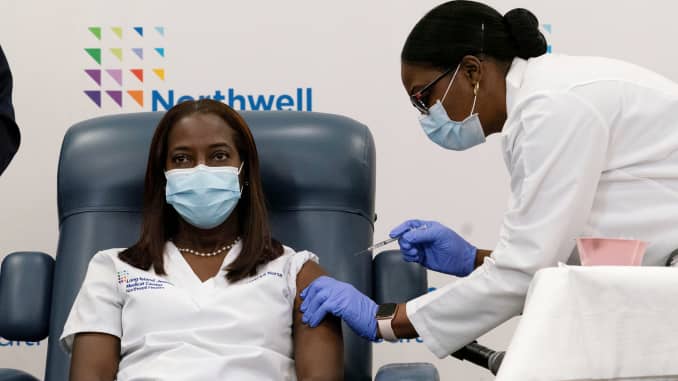
New York critical care nurse, Sandra Lindsay, receiving the Pfizer-BioNTech COVID-19 vaccine. Image retrieved from CNBC.
December 14, 2020
At 6:31 a.m. on Dec. 8, 2020, 334 days after the first reported COVID-19 death in China, Margaret Keenan, 90, became the first person in the world to receive the clinically approved Pfizer-BioNTech vaccine at University Hospital in Coventry, a city northwest of London.
Six days earlier, Britain gave Emergency Use Authorization to the vaccine, becoming the first Western country to allow mass inoculations. According to a United Kingdom correspondent for The New York Times Benjamin Mueller, “Britain’s beating the United States to authorization — on a vaccine co-developed by an American company, no less — intensified pressure on U.S. regulators, who are under fire from the White House for not moving faster to get doses to people.”
The increasing number of cases, especially in November, heightened the United States’ need for a vaccine. “When you say it’s ok to wait another week or two, you’re saying it’s ok that many thousands of people are going to die,” said Dr. Walid F. Gellad, who leads the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh.
The federal government, through Operation Warp Speed, has been working to make one or more COVID-19 vaccines available as soon as possible. According to the U.S. Department of Health and Human Services, “Operation Warp Speed’s goal is to produce and deliver 300 million doses of safe and effective vaccines with the initial doses available by January 2021, as part of a broader strategy to accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics.”
On Dec. 11, 2020, the FDA released a statement issuing Emergency Use Authorization (EUA) for the first COVID-19 vaccine. While not an FDA approval, the authorization provides hope to the nation that the end of the pandemic could be in sight.
According to the FDA in a news release, “The totality of the available data provides clear evidence that Pfizer-BioNTech COVID-19 Vaccine may be effective in preventing COVID-19. The data also support that the known and potential benefits outweigh the known and potential risks, supporting the vaccine’s use in millions of people 16 years of age and older, including healthy individuals. In making this determination, the FDA can assure the public and the medical community that it has conducted a thorough evaluation of the available safety, effectiveness, and manufacturing quality information.”
Before Pfizer’s COVID-19 vaccine proved highly successful in clinical trials in November with an effectiveness rate of 95% after the first dose, the company offered the Trump administration the chance to lock in supplies beyond the 100 million promised doses as part of a $1.95 billion deal with the U.S. government over the summer. However, the White House administration did not make the deal, which may slow down the overall distribution of the vaccine over 2021.
While Pfizer is currently negotiating with the administration to provide more of its vaccine, the company cannot guarantee that it will be able to deliver more than the initial 100 million doses before next June. This would be enough to inoculate 50 million people since its vaccine requires two shots spread a few weeks apart.
Two other companies that may be able to aid in vaccine development and distribution include Moderna and AstraZeneca. The Moderna vaccine will be reviewed by an expert panel Thursday, Dec. 17, and soon afterward could be allowed for public use.
On Dec. 13, the first vaccine shipments left the Pfizer Global Supply Kalamazoo manufacturing plant in Portage, Michigan, beginning the nationwide rollout of the Pfizer-BioNTech vaccine within the United States. The initial shipments contain about 3 million doses, with many more to follow. This is expected to be the biggest vaccination effort in U.S. history.
On Dec. 14, 2020, COVID-19 vaccinations were given to Americans in mass for the first time, beginning at 9 a.m. with critical care nurse, Sandra Lindsay, at New York’s Long Island Jewish Medical Center. The administration of the Pfizer-BioNTech vaccine arrived after 300,000 people died nationwide from the virus.
According to the chief operating officer of the federal effort to develop a vaccine, Gen. Gustave F. Perna, 145 sites were set to receive the vaccine on Monday, 425 on Tuesday, and 66 on Wednesday. A majority of the first injections are expected to go to high-risk health care workers and residents of nursing homes.
In an interview with MSNBC on Dec. 14, the nation’s top infectious disease expert, Dr. Anthony S. Fauci, predicted that the average person with no underlying conditions would get the vaccine by the end of March or beginning of April. He said that if the campaign is efficient and effective, most Americans could be vaccinated with the first dose by late spring or early summer.
The same day at 2:30 p.m. at UW Health in Madison, Wisconsin, respiratory therapist Tina Schubert became the first UW Health employee and one of the first Wisconsin residents to be inoculated with the Pfizer-BioNTech vaccine.
“Wisconsin expects to receive 49,725 doses of the Pfizer vaccine this week. Soon after, the state should receive about 101,000 doses of the COVID-19 vaccine made by Moderna as long as the company receives emergency use authorization from the U.S. Food and Drug Administration. […] Widespread availability of the vaccines is still months away.”
Although the COVID-19 vaccine will become increasingly available to the average American in 2021, many still have apprehensions about vaccination. According to journalists for Associated Press, Lauran Neergard and Hannah Fingerhut, “A survey from The Associated Press-NORC Center for Public Affairs Research found that about half of Americans want to get the vaccine as soon as possible. Another quarter aren’t sure, while the remaining quarter says they aren’t interested. Some simply oppose vaccines in general. Others are concerned that the vaccines have been rushed and want to see how the rollout goes.”
The cost will not be an obstacle to vaccination. According to the CDC, “Vaccine doses purchased with U.S. taxpayer dollars will be given to the American people at no cost. However, vaccination providers may be able to charge administration fees for giving the shot. Vaccination providers can get this fee reimbursed by the patient’s public or private insurance company or, for uninsured patients, by the Health Resources and Services Administration’s Provider Relief Fund.”
According to the CDC, although there may be minor flu-like symptoms after receiving the COVID-19 vaccine, it is a normal sign that your body is building protection. The CDC ensures that the safety of COVID-19 vaccines is a top priority. Clinical trials are evaluated investigational COVID-19 vaccines in many thousands of study participants to generate scientific data.